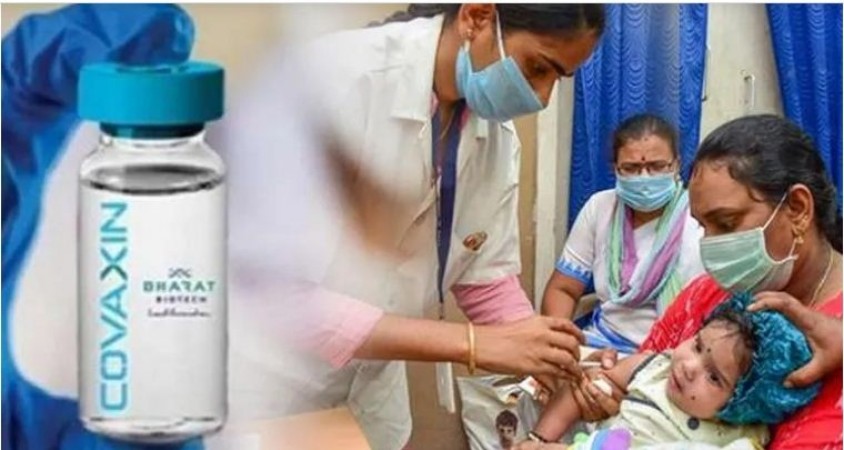
The Central government and the Drugs Controller-General of India (DCGI) may give approval to the Covaxin inoculation for children soon for emergency use, sources said. The administration of two doses of vaccination has been completed among children and blood samples have been sent for the third time to check the effectiveness in terms of producing antibodies among children.
A total of 90 children were subjected to Covaxin trials in Karnataka. However, the trial will take 210 days to complete. As it will take another 5 to 6 months from now, they can't wait till then in the current circumstances to release the vaccination for children, sources underlined. "Final report will come on 210th day.
Before also, while releasing Covid vaccines for adults the government of India and DCGI did not wait till the completion of 210 days. Vaccines were released earlier," sources said. Zydus Cadila was recently authorized by DCGI for its needle-free ZyCoV-D Covid-19 vaccine for children aged 12 years and above. The government and DGCI can take calls any day after the 56th day of the trial Emergency use. The DGCI might take a call for the release of Covid vaccines for the children after ensuring efficacy of vaccines through antibody titre tests, reliable sources in the health department underlined.
Easing curbs, Karnataka State Transport to restart bus services to Tamil Nadu from today
"India to vaccinate Afghan returnees against polio": Union Health Minister Mansukh Mandaviya
Bullet Train To Run Between New Delhi and Ayodhya: It's official