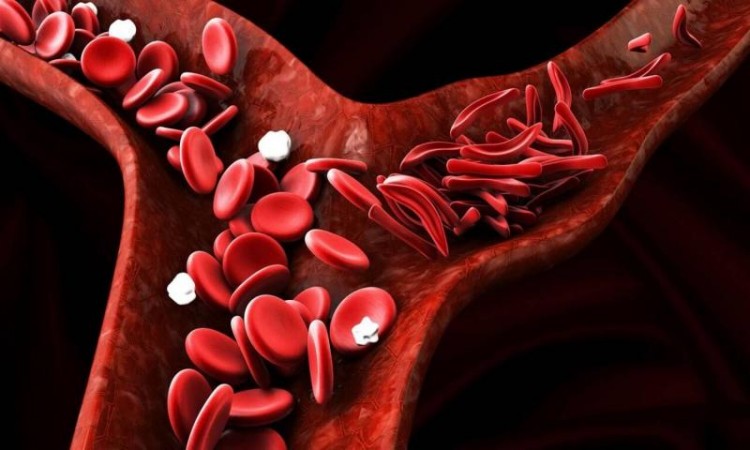
HYDERABAD: The Drugs Controller General of India (DCGI) has approved the use of hydroxyurea to treat Sickle Cell Anemia.
The CSIR-Sickle Cell Anaemia (CSIR-SCA) Mission has sought the DCGI for clearance, organised by the CSIR-Centre for Cellular and Molecular Biology (CSIR-CCMB) with the help of Cipla, a hydroxyurea manufacturer, and with active support from CSIR-IIIM.
According to the CCMB on Thursday, a committee of specialists appointed by the Central Drug Standard Control Organisation (CDSCO) critically assessed the proposal and approved the marketing of hydroxyurea for the treatment of SCA, subject to post-marketing surveillance.
The medicine can now be used to treat SCA at regular doses, thanks to the authorisation. It also paves the way for the development of smaller-dose formulations that promise higher compliance rates in SCA youngsters, and could potentially lead to syrup-based formulations, according to the CCMB.
"For the sickle cell anaemia community, this is a watershed moment. This further enhances the benefits of identifying patients through a tailored screening programme. While one of the primary goals of the screening programme is to prevent the birth of afflicted children through genetic and social counselling, this approval allows individuals to receive full treatment.
The CSIR-SCA Mission is being led by Dr Giriraj R Chandak, Chief Scientist at CSIR-CCMB and Mission Director.
India's COVID-19 vaccination coverage surpasses 140.31 cr
Government directs local level restrictions on sudden rise in Omicron patients across the country
Oxford, AstraZeneca developing Omicron-targeted variant of vaccine