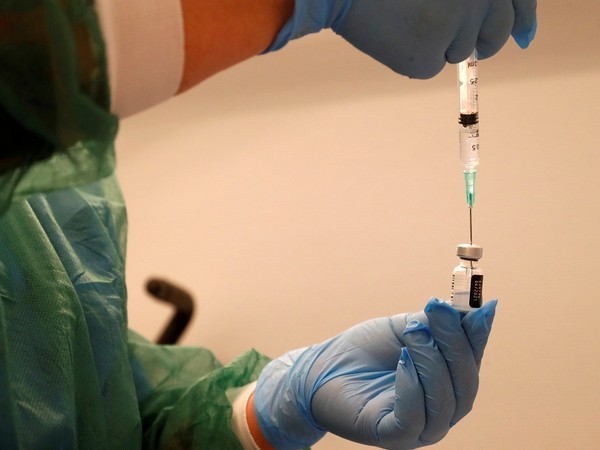
The European Commission on Friday approved a conditional marketing authorisation (CMA) for the corona vaccine developed by AstraZeneca.
The Commission granted conditional marketing authorisation for the AstraZeneca's COVID-19 vaccine. In a statement, the commission said, "Today, the European Commission has granted a conditional marketing authorisation (CMA) for the COVID-19 vaccine developed by AstraZeneca, the third COVID-19 vaccine authorised in the EU." The commission said that the AstraZeneca vaccine will be given to adults aged 18 years and older for preventing corona.
Stella Kyriakides, Commissioner for Health and Food Safety, said, "The European Medicines Agency's authorisation today is another step towards delivering on this promise. The Commission continues to work around the clock to secure more vaccines for Europe and our international partners. We are leaving no stone unturned in our fight against this pandemic."
Talking about corona cases, Coronavirus cases rise unabated across the globe, with nearly 102.6 million infected by the deadly contagion. While 74,299,138 have recovered, 2,214,227 have died so far. However, it terms of the total number of active cases, US tops the charts, followed by France, UK, Brazil and Belgium.
Also Read:
Colombia's corona deaths toll reaches 53,000
Israel Embassy official to India: Delhi blast could be 'terror attack'
Sriwijaya Air plane Crash: Indonesian officials identify body of pilot