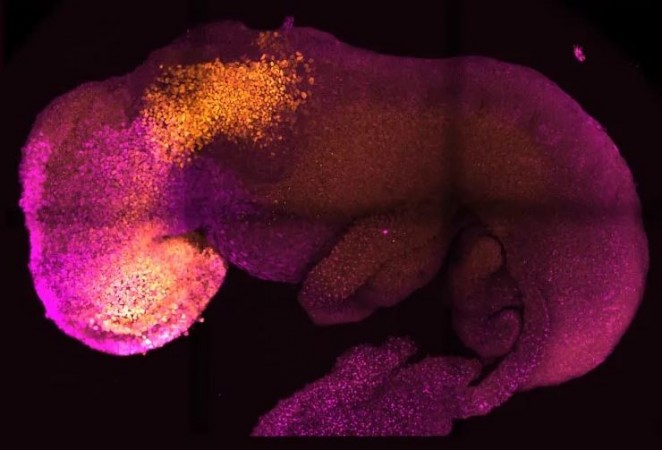
With the help of mouse stem cells, researchers from the University of Cambridge have built model embryos with a brain, a heart that beats, and the building blocks for every other organ in the body. It represents a fresh way to recreate the beginnings of life.
The embryo model was created by the study team without the use of eggs or sperm, under the direction of Professor Magdalena Zernicka-Goetz. Instead, they made use of stem cells, which are the body's master cells and may differentiate into practically any form of cell.
The researchers imitated natural processes in the lab by directing the three varieties of stem cells that are present in early mammalian development to the point where they begin interacting. By increasing the expression of a certain set of genes and creating a special environment for their interactions, the researchers were able to induce the stem cells to "speak" to one another.
The stem cells self-organized into structures that developed through the various phases of development until they had beating hearts and the brain's structural components. The yolk sac, where the embryo grows and receives nutrition during its first few weeks, was also present. The Cambridge-developed models, in contrast to earlier synthetic embryos, progressed to the stage where the complete brain, including the anterior region, began to form. In comparison to other stem cell-derived models, this represents a further stage of development.
The team believes that their findings could aid in the understanding of why some embryos do not grow into healthy pregnancies while others do. The outcomes could also be utilised to direct the production and repair of artificial human organs for transplantation. The study was published on August 25, 2022, in the magazine Nature. It is the outcome of more than ten years of research that has steadily produced increasingly complicated embryo-like structures.
“Our mouse embryo model not only develops a brain, but also a beating heart, all the components that go on to make up the body,” said Zernicka-Goetz, Professor in Mammalian Development and Stem Cell Biology in Cambridge’s Department of Physiology, Development and Neuroscience. “It’s just unbelievable that we’ve got this far. This has been the dream of our community for years, and major focus of our work for a decade and finally we’ve done it.”
A healthy development of a human embryo requires a "dialogue" between the tissues that will create the embryo and the tissues that will connect the embryo to the mother. In the first week after fertilisation, three distinct stem cell types start to emerge; one of these will eventually develop into the body's tissues, while the other two aid in the growth of the embryo. The placenta, which connects the foetus to the mother and supplies oxygen and nourishment, will develop from one of these extraembryonic stem cell varieties. The yolk sac is the second, where the embryo develops and receives its initial nutrition.
When the three different stem cell types start communicating with one another and giving instructions to the embryo on how to develop appropriately, many pregnancies end prematurely.
“So many pregnancies fail around this time, before most women realize they are pregnant,” said Zernicka-Goetz, who is also Professor of Biology and Biological Engineering at Caltech. “This period is the foundation for everything else that follows in pregnancy. If it goes wrong, the pregnancy will fail.”
Over the past ten years, the Cambridge research team of Professor Zernicka-Goetz has been examining these first few weeks of pregnancy in an effort to comprehend why some pregnancies end in failure and others in success.
“The stem cell embryo model is important because it gives us accessibility to the developing structure at a stage that is normally hidden from us due to the implantation of the tiny embryo into the mother’s womb,” said Zernicka-Goetz. “This accessibility allows us to manipulate genes to understand their developmental roles in a model experimental system.”
The researchers assembled cultivated stem cells that represented each of the three tissue types in the proper ratios and environments to encourage their growth and communication with one another, finally self-assembling into an embryo.
In order to guide the development of the embryo, extraembryonic cells communicate with embryonic cells not just chemically but also mechanistically, or through touch.
“This period of human life is so mysterious, so to be able to see how it happens in a dish – to have access to these individual stem cells, to understand why so many pregnancies fail and how we might be able to prevent that from happening – is quite special,” said Zernicka-Goetz. “We looked at the dialogue that has to happen between the different types of stem cell at that time – we’ve shown how it occurs and how it can go wrong.”
The capacity to synthesise the full brain, especially the anterior portion, which has been a primary aim in the development of synthetic embryos, represents a significant advancement in the field. Because this area of the brain needs signals from one of the extraembryonic tissues to develop, this is effective in Zernicka-technique. Goetz's From their 2018 and 2021 trials, which used the same component cells to develop into embryos at a somewhat earlier stage, the scientists hypothesised that this might be happening. They can now declare with certainty that their model is the first to indicate growth of the anterior and in fact the entire brain, by delaying development by just one day.
“This opens new possibilities to study the mechanisms of neurodevelopment in an experimental model,” said Zernicka-Goetz. “In fact, we demonstrate the proof of this principle in the paper by knocking out a gene already known to be essential for the formation of the neural tube, the precursor of the nervous system, and for brain and eye development. In the absence of this gene, the synthetic embryos show exactly the known defects in brain development as in an animal carrying this mutation. This means we can begin to apply this kind of approach to the many genes with unknown function in brain development.”
While the current study used mouse models, analogous human models are being created by researchers with the capacity to generate certain organ types. This will help them understand the mechanisms underlying critical processes that would otherwise be hard to investigate in real embryos. Up to the 14th day of development, human embryos may only be researched in the lab under current UK law.
The techniques created by Zernicka-team Goetz's could also be used to direct the creation of synthetic organs for patients awaiting transplants if they are later demonstrated to be effective with human stem cells.
“There are so many people around the world who wait for years for organ transplants,” said Zernicka-Goetz. “What makes our work so exciting is that the knowledge coming out of it could be used to grow correct synthetic human organs to save lives that are currently lost. It should also be possible to affect and heal adult organs by using the knowledge we have on how they are made.
“This is an incredible step forward and took 10 years of hard work of many of my team members – I never thought we’d get to this place. You never think your dreams will come true, but they have.”
Can Covid-19 be eliminated? no not at all according to CDC chief Dr. Anthony Fauci
US and Chinese scientists discover an easy method to eliminate harmful "forever chemicals"
US aimed scientist from China for "discovering the world's best semiconductor"