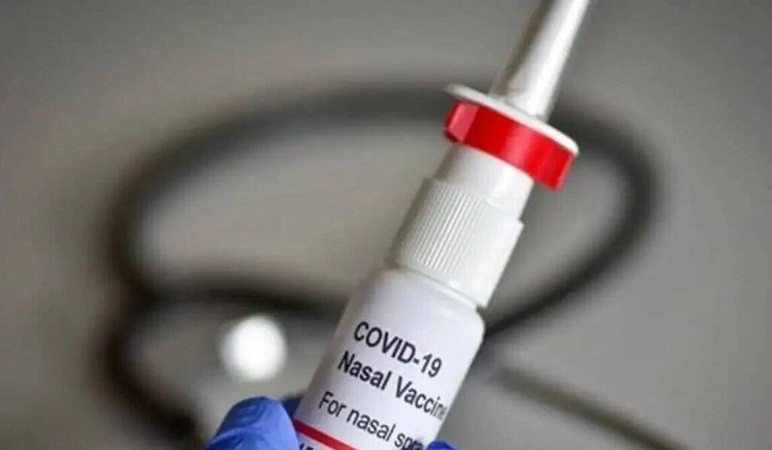
Bharat Biotech said on Tuesday that its nasally administered vaccine namely, iNCOVACC, would be available for public use from the 4th week of January. The Nasal vaccine cost Rs.800 for private markets and Rs. 325 for governments outlets.
iNCOVACC, the first intranasal vaccine for COVID-19 to receive approval for both the primary two-dose schedule and a heterologous booster dose, will be administered as a booster dose to people over the age of 18.
Washington University in St. Louis, Missouri, produced the recombinant adenoviral-vectored construct and tested its efficacy in preclinical tests before designing and developing iNCOVACC. The nasal vaccination, unlike Covaxin, contained only a portion of the SARS-CoV-2 virus, specifically the spike protein, and was encased in a virus that is normally non-lethal to humans.
Bharat Biotech carried out product development activities such as preclinical safety assessment, large-scale manufacturing scale-up, formulation and delivery device development, and human clinical trials. The COVID Suraksha Programme of the Department of Biotechnology was used by the Government of India to partially support product development and clinical testing.
Mansukh Mandaviya, the union minister of health, announced on Friday that inCOVACC has been given the go-ahead for usage by the general public. On the COWIN website, appointments can be made for the booster shot.
Covid-19: Andhra holds review meeting for village clinics
Centre working towards elimination of TB by 2025: Dr Bharati Pawar
India reports 157 new Covid cases in 24-hours