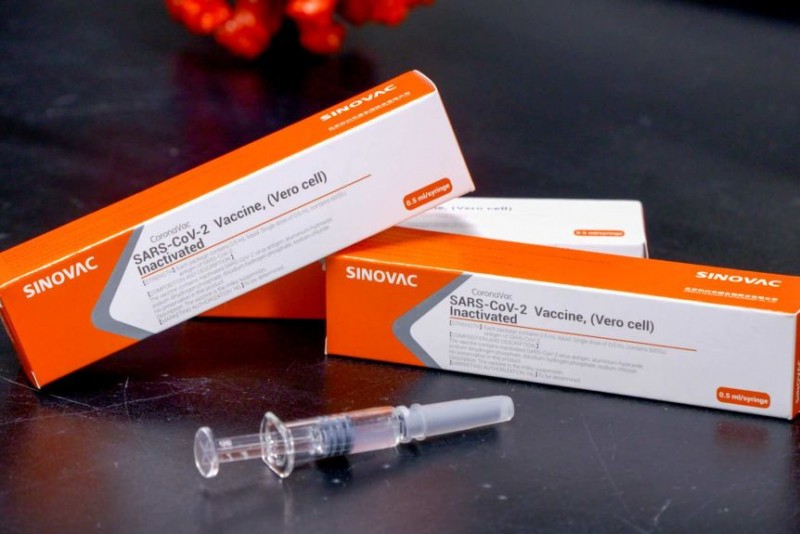
In China, the use of a drug covid-19 has been approved in emergency. Synovac Biotech Ltd's covid-19 drug was approved in July. Synovac's use of the corona drug Coronavac in emergency has been approved. A person associated with the case said that the vaccine is being used in China as an emergency in China as part of a program under which this drug will be applied to high-risk teams such as medical staff.
The Pharmaceutical Group (Sinopharm), owned by the China National Biotech Group, also stated that it has obtained approval for emergency use. CNBG, whose two vaccines are currently in Phase III trials. However, it was not disclosed which of the 2 vaccines have been approved for use in emergency.
A Chinese health officer said in an interview aired last week that China has been giving experimental corona virus vaccines to high-risk groups since July. Officially, China has given little details on which vaccine. Under this, candidates have been given a dose of vaccine for high risk people. How many more people have been in its emergency use program. A Chinese health official said that China has approved the use of the corona vaccine developed by a few select domestic companies, saying that they can use it in emergency situations. Let me tell you that the trial of corona vaccine is currently going on in different stages in the country, but before this the emergency use of the vaccine has been approved in the country.
Also Read:
Students will be able to study in European countries again!
Over 5000 people arrested in China due to coronavirus
Mexico: Over 516 patients lost their lives in 24 hours due to coronavirus
Japan PM Shinzo Abe resigns quoting health issues