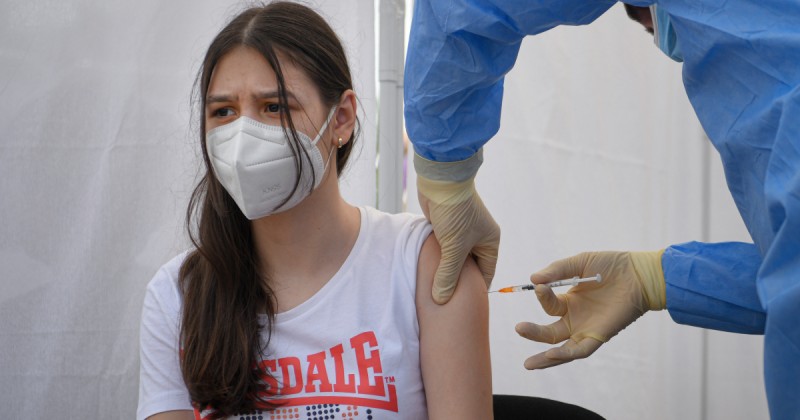
The European Medicines Agency has approved use of Moderna's Covid vaccine for children aged between 12 and 17. It is the second Covid jab to be approved for adolescents by the EU's medicines authority - in May, the Pfizer-BioNTech one got the go-ahead. The US-made Moderna jab requires two doses, four weeks apart, the EMA says.
The European Commission is expected to widen vaccination with Moderna to young people now, based on the EMA's recommendation. But the move is likely to have its critics, as the vaccination rate in most of Asia and Africa - where millions of adults risk serious illness or death from Covid - is far lower than in the EU, where more than half of adults are now fully vaccinated. The World Health Organization has urged wealthy countries to spread vaccines much more widely globally.
However, supporters argue widening vaccination to children is important now that the highly infectious Delta variant is widespread in Europe and cases are rising sharply in some areas. Children also have to be included if the goal of herd immunity is to be reached - that is, when a high level of vaccination slows the virus's spread in the population.
A far-right extremist killed 77 people in Norway. A decade on, 'the hatred is still out there'
Covid Crisis: South Africa goes ahead to postpone local govt polls
Bangladesh heads into most severe lockdown to curb the raging spread
Biden poised to nominate Caroline Kennedy as US ambassador to Australia