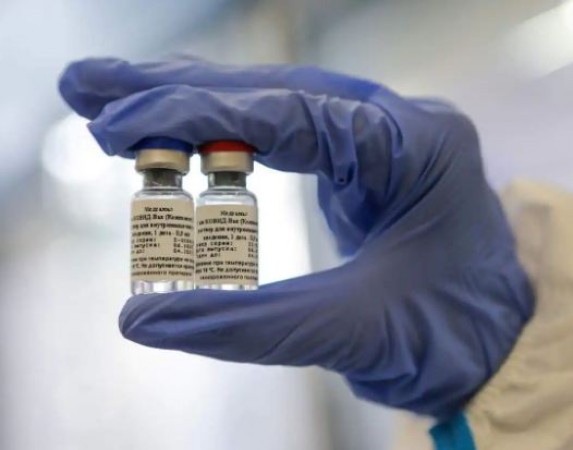
New Delhi: Attempts to introduce the Corona vaccine Sputnik-V in India, Russia has suffered a major setback. The regulatory body has returned the proposal of Dr. Reddy's Laboratories on a large scale for human testing. Hyderabad's pharma company wanted to do a large scale human trial in India to evaluate the Russian vaccine.
Experts from the Central Drugs Standard Control Organization (CDSCO) said that Russia's coronavirus vaccine should be tested on a small group first. The recommendations of the expert panel acknowledge that immunogenicity and safety data of early-stage testing abroad are small and there is no vaccine input available on Indian participants. Russia's Direct Investment Fund (RDIF) and Dr. Reddy's Laboratories last month announced a partnership to conduct human trials and distribute vaccines in India.
Earlier, the regulatory body had asked Dr. Reddy's Laboratories to apply again. The panel of CDSCO had said that Dr. Reddy's would have to introduce a revised protocol for the second and third phase of human trials of Sputnik-V. Along with this, the Hyderabad-based pharma company was also asked to provide some other information.
PM Modi expresses grief over Ram Vilas Paswan's untimely demise
Donald Trump to hold a promotional event in Florida on this day