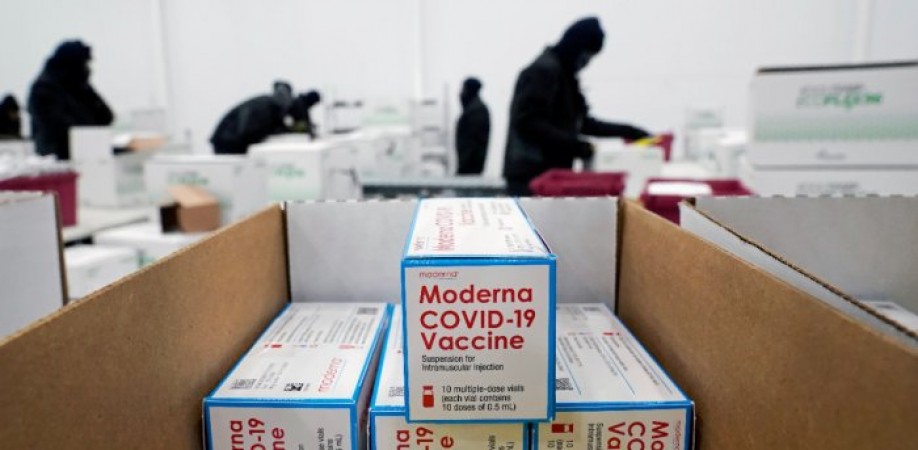
United States Food and Drug Administration has approved the use of Moderna as its second vaccine for Emergency use Approval. Employees at a factory in the Memphis area were boxing up the vaccine developed by Moderna Inc. and the National Institutes of Health. The much-needed vaccine shots are expected to be given starting Monday, just three days after the Food and Drug Administration authorised the vaccine emergency rollout.
An expert committee will debate over who should be next in line for early doses of the Moderna vaccine and a similar one from Pfizer Inc. and Germany's BioNTech. Public health experts say the shots and others in the pipeline are the only way to stop a virus that has been spreading in an uncontrolled manner. Across United States more than 219,000 people per day on average test positive for the virus, which has killed more than 314,000 in the US and nearly 1.7 million worldwide.
The experts say there won't be enough shots for the general population until spring, so doses will be rationed at least for the next several months. The panel members are thinking to put the "essential workers" next in line, because people like bus drivers, grocery store clerks and others are the ones getting infected most often. But few other experts say people who are 65 and older should be next, along with people with certain medical conditions, because those are the Americans who are dying at the highest rates. Both Moderna nad Pfizer requires two doses and the vaccines appeared safe and strongly protective in large, still unfinished studies.
US approves Moderna as second vaccine for COVID-19
US to buy 200 million doses of Moderna, first set of 20M to be delivered in December