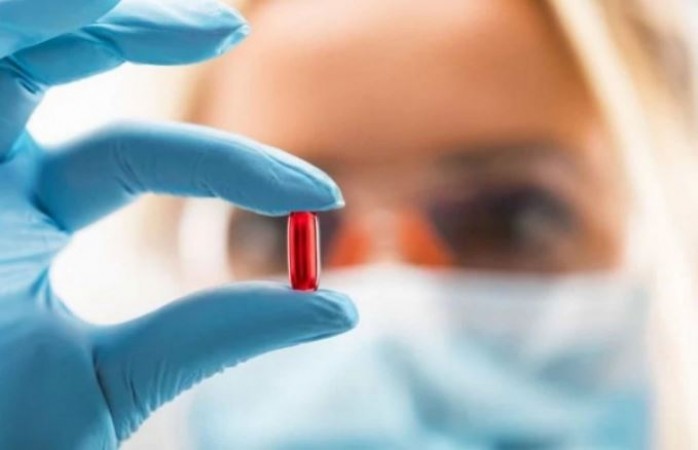
The hetero company has sought permission from the DCGI for emergency use of the coronavirus drug Molnupiravir. Molnupiravir Tablet and can be given to mild patients. The hetero company claims based on clinical trials that it can eliminate infections from the corona patient's body in 5 days.
In the same third phase of the interim trial, 1218 patients have been asked for permission for emergency use of the drug after trial. This medicine has been prepared by Merck and Ridgeback Biotherapeutics LP. This medicine will be effective for patients who are not hospitalized and are undergoing treatment at home. The interim result has been based on 714 patients.
Claims to end infection from the body in 5 days:-
Earlier, it was reported that the pharmaceutical company Merc had announced an agreement with five generic drug manufacturers in India to produce experimental antiviral tablets that are considered similar to the corona drug Remdesivir. A study on the drug showed encouraging results, which showed that its intake immediately after infection leads to a drop in the level of infection from the rafter.
Corona conditions in the country:-
Coming to the situation in Corona across the country, according to data released by the Union Health Ministry on Friday morning, 43,393 new cases have been reported in the last 24 hours, while 911 people have lost their lives. At present, there are 4,58,727 active cases in the country, which is 1.49% of the total cases reported so far.
Will Mamata send Sourav Ganguly to Rajya Sabha? Speculation intensifies after they met
Pinarayi Vijayan calls for PM Modi to waive duty on life-saving drug for kids
Schools to open soon in this state, school education minister says