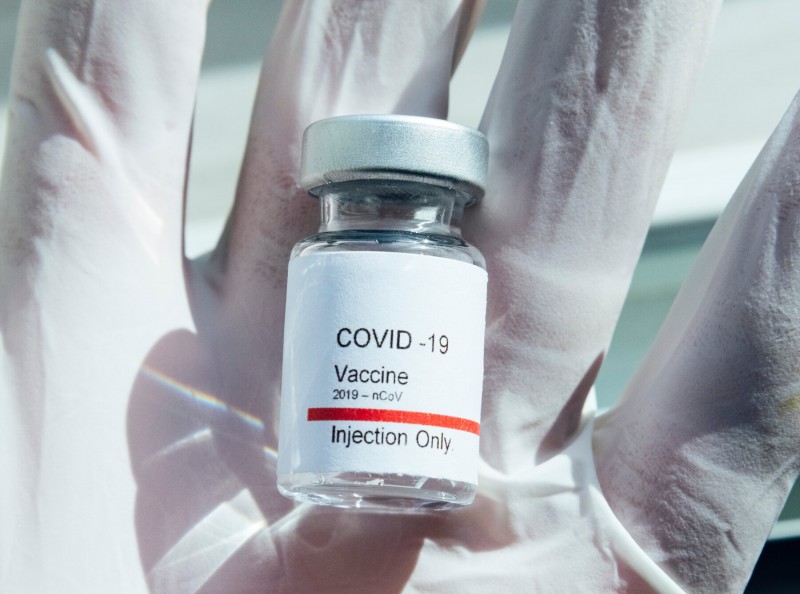
The news regarding vaccines are gaining momentum daily. Russia has been given approval by the Drug Control General of India (DGCI) to conduct late-stage clinical trials of Sputnik V COVID-19 vaccine in India. Distinctly, this will be a multi-center and randomized controlled study, which will include safety and immunogenicity study, pharmaceutical major Dr. Reddy’s Laboratories Ltd said in a press release.
700 Students in a college tested COVID-19 positive, the college president resigns
Previously, Dr.Reddys Laboratories Ltd. and the Russian Direct Investment Fund (the official sponsor of the vaccine candidate) had joined into a partnership to conduct clinical trials of Sputnik V vaccine and its distribution in India. As part of the partnership, RDIF shall supply 100 million doses of the vaccine to Dr.Reddy’s regulatory approval in India. G V Prasad, Co-chairman and Managing Director, Dr.Reddy, said “This is a significant development that allows us to commence the clinical trial in India and we are committed to bringing in a safe and efficacious vaccine to combat the pandemic.”
New Zealand PMJacinda Ardern party made a remarkable victory in the general election
Kirill Dmitriev, CEO of the Russian Direct Investment Fund, stated, “We are pleased to collaborate with the Indian regulators and in addition to Indian clinical trial data, we will provide safety and immunogenicity study from the Russian phase 3 clinical trial. This data will further strengthen the clinical development of Sputnik V vaccine in India.” Sputnik V, an adenovirus vector-based vaccine, was developed by the Gamaleya Scientific Research Institute of Epidemiology and Microbiology, along with the Russian Direct Investment Fund and registered on August 11.
Canadian city Winnipeg shuts its bars and Restros in the wake of increased cases of coronavirus