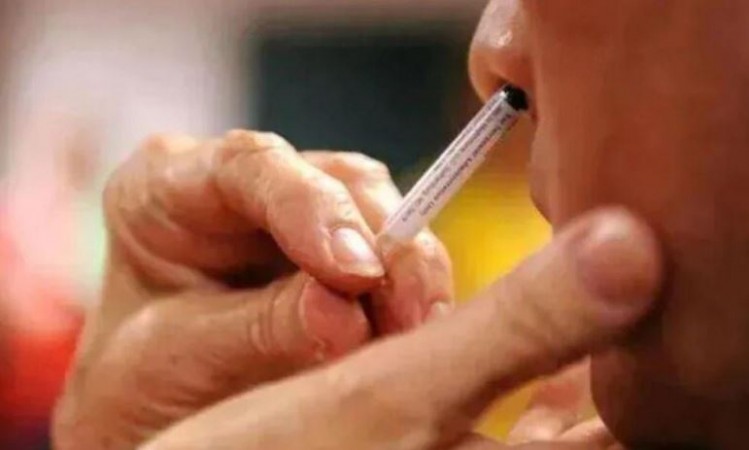
New Delhi: Amid the increasing cases of corona all over the world, the central government has approved the use of Bharat Biotech's nasal vaccine. This vaccine will be available as a booster dose. Explain that this nasal vaccine will initially be available in private hospitals. Earlier, the Drug Controller General of India (DCGI) had given permission for the emergency use of Bharat Biotech's intranasal COVID vaccine.
This vaccine is given by spraying through the nose, i.e. the vaccine is not given on the arm of the recipient. DCGI has approved the intranasal Covid vaccine for people above 18 years of age. The name of this vaccine of Bharat Biotech is BBV154. Hyderabad-based Bharat Biotech has conducted clinical trials of the nasal vaccine on 4000 volunteers. It has not shown any side effects on any of these volunteers.
After the phase 3 clinical trial in August, it was clear that the BBV154 vaccine is safe for use. Regarding BBV154, Bharat Biotech has informed that this vaccine is given through the nose. It has also been said that this vaccine is affordable which will be right for low and middle-income countries. It has been told that this vaccine will work to reduce infection and illness.
These important decisions were taken in the meeting regarding Sammed Shikhar
Corona arrived with the new year, will the celebration fade again?
Bharat Jodo Yatra reaches NCR, Rahul will reach Delhi tomorrow