Washington: An eagerly awaited new medication to slow cognitive decline in people with early-stage Alzheimer's disease has been approved by the US Food and Drug Administration.
Just a few days prior to the FDA approving the drug Leqembi, also known as lecanemab, the organisation had received harsh criticism in a congressional report for approving the drug Aduhelm, another treatment for Alzheimer's.
And yet, despite trial results indicating that there is a risk of brain bleeding and swelling with monoclonal antibody therapy, it was still approved.
Also Read: 6-year-old boy shot a female teacher, hospitalized in a critical condition
In order to expedite the approval of medicines for serious conditions where there is a medical need, the FDA approved both drugs using an expedited procedure.
The FDA stated in a statement that Leqembi and Aduhelm, both of which were jointly created by the American pharmaceutical company Biogen and Japan's Eisai, "represent an important advancement in the ongoing fight to effectively treat Alzheimer's disease."
According to Billy Dunn of the FDA's Center for Drug Evaluation and Research, "Alzheimer's disease immeasurably impairs the lives of those who suffer from it and has devastating effects on their loved ones."
Leqembi is "the most recent treatment to target and affect the underlying disease process of Alzheimer's, rather than only treating the disease's symptoms," according to Dunn.
Leqembi showed a 27 percent reduction in the rate of cognitive decline in Alzheimer's patients, according to preliminary results from a trial that were published in September.
Over the course of 18 months, the phase three trial included nearly 1,800 participants who were split between receiving the drug and a placebo.
Also Read: In preparation for Russia sanctions, Abramovich's trusts were reorganised.
The full trial data, which was published in the New England Journal of Medicine, provided more detail on the results but also raised questions about how frequently "adverse effects," such as brain bleeding and swelling, occur.
According to the findings, 17.3% of patients who received the medication experienced brain bleeds, compared to 9% of patients who received a placebo.
Additionally, 12.6% of those taking the medication experienced brain swelling, as opposed to just 1.7% of those receiving a placebo.
In both arms of the drug trial, deaths were reported at roughly the same rates.
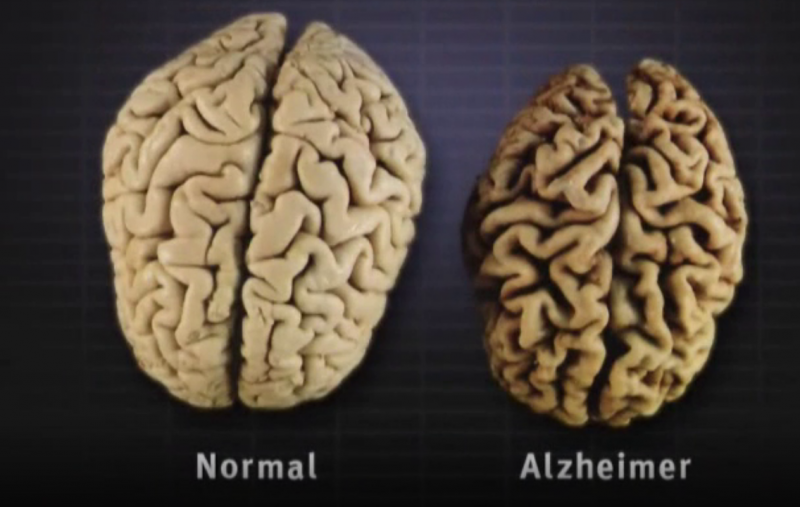
In Alzheimer's disease, two essential proteins, tau and amyloid beta, accumulate into tangles and plaques, collectively known as aggregates, which result in the death of brain cells and subsequent brain atrophy. Leqembi functions by going after amyloid.
Aduhelm was previously marketed by Biogen and Eisai, but there was considerable debate over its efficacy, and its approval in 2021 resulted in three high-level FDA resignations.
Also Read: US judge chastises Trump attorneys and rejects motion to dismiss New York fraud case
Aduhelm's approval procedure was "rife with irregularities," according to an 18-month US congressional investigation, which also criticised the agency and Biogen.
According to the congressional report, the Cambridge, Massachusetts-based Biogen set a "unjustifiably high price" of $56,000 annually for Aduhelm.
Leqembi's initial cost, according to Eisai, will be $26,500 annually.