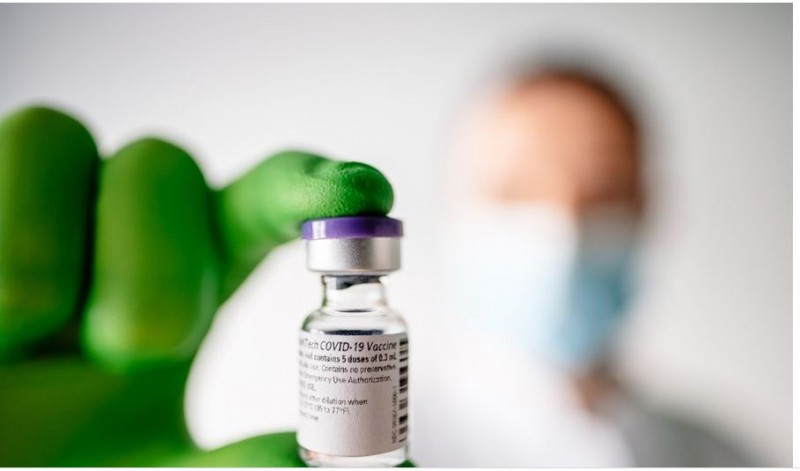
Washington: The US Centers for Disease Control and Prevention (CDC) advisory panel on Thursday endorsed booster doses of the Pfizer-BioNTech COVID-19 vaccine for Americans aged 65 and older and some adults with certain high-risk conditions.
The endorsement comes a day after the Food and Drug Administration granted emergency use authorization to administer third Pfizer shots to many Americans six months after they complete their first two doses. While the CDC’s committee’s recommendation isn’t binding, Walensky is expected to accept the panel’s endorsement shortly.
The panel voted 15-0 to recommend a booster dose for Americans age 65 and older and people in long-term care facilities. It also fully recommended giving a single booster dose to people between the ages of 50 and 64 with certain high-risk conditions, by a vote of 13-2.
On Wednesday, the Food and Drug Administration approved Pfizer boosters for people aged 65 and older or at high-risk. The extra dose would be given once they are at least six months past their last Pfizer shot. CDC Director Rochelle Walensky is expected to accept the panel's endorsement shortly.
World Bank agrees to give USD 100mn to Sri Lankafor Covid battle
Land-sea trade corridor links China, Vietnam
Thailand plans to postpone reopening of key tourist destinations until November