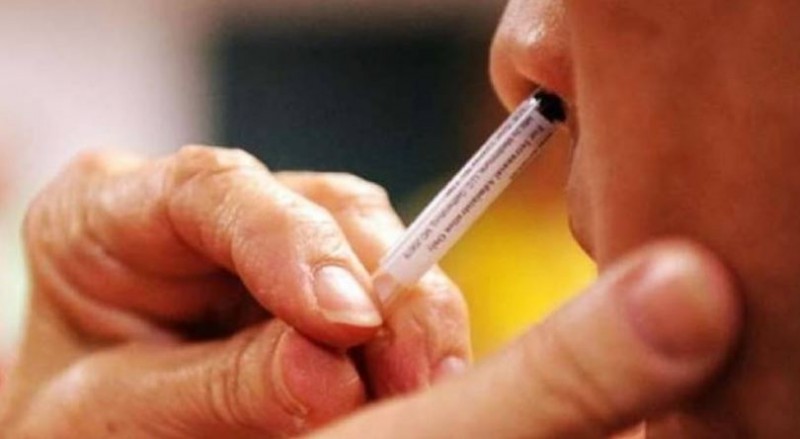
New Delhi: A 2/3 round clinical trial of India Biotech's Nasal Corona vaccine will be held soon at AIIMS, Delhi. According to sources, the trial of the Nasal vaccine will begin in a few weeks. The Covaxin of Bharat Biotech was also trialed in AIIMS. India Biotech's intranasal vaccine has received regulatory approval for the second phase of testing.
At the same time, the AIIMS ethics committee has been sent for approval for conducting this clinical trial. Dr. Sanjay Rai will be the principal investigator of the trial of the vaccine to be given in the nose. For the clinical trial, permission has to be obtained from the Ethics Committee of AIIMS Hospital for which Bharat Biotech has applied.
Companies around the world are working on the Nasal spray vaccine to be given. The Nasal vaccine is believed to be more effective and may prove to be a game-changer. There are many benefits of giving the vaccine through the nose, the first being that it will eliminate the need for needles (syringes), which will reduce the risk of injury and infection. Apart from this, administering these vaccines is also easy. Many of these Nasal vaccines are also being trialed for children. If it is successful, it will be a big relief.
Threat of third wave! 532 new cases in Mumbai
UP Dengue: Over 30 cases reported in Meerut, admin initiates preventive action plan
Taliban again in Mehbooba Mufti's heart, people said- 'Send her to Afghan'