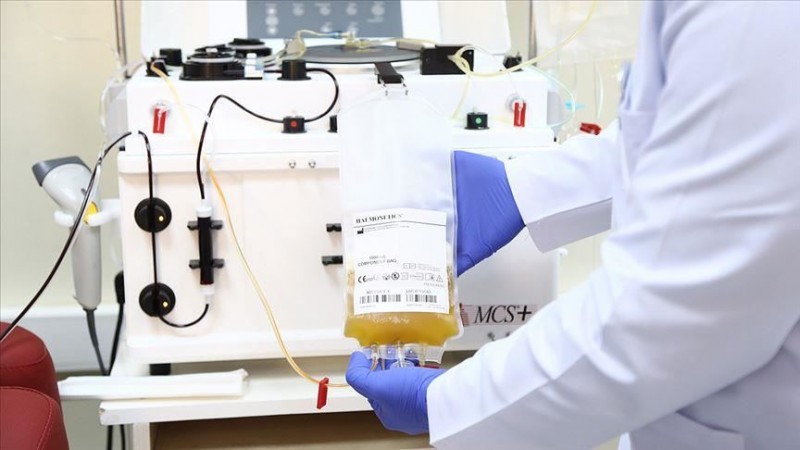
Japan’s Takeda Pharmaceutical Co said on Friday an alliance of drugmakers it spearheads has enrolled its first patient in a global clinical trial of a blood plasma treatment for COVID-19 after months of regulatory delays. 500 adult patients from various part of the globe including the United States, Mexico and 16 other countries are targeted for the phase3 trails by the Takeda Pharma Co group, phase3 trails are known as COVIg Plasma Alliance.
Patients will be treated with Gilead Science Inc’s Remdesivir alongside the plasma treatment, which will be provided by CSL Behring, Takeda and two other companies. “We are hopeful that data from the clinical trial will be available before the end of the year,” said Bill Mezzanotte, chief medical officer of CSL Behring. The group planned to begin the trail by July 2020 but gets delayed due to the pending regulatory approval. The sponsor for this phase 3 trial is the National Institutes of Health in the U.S.
The alliance, which also includes Germany’s Biotest AG and Octapharma Plasma, is working on a hyperimmune globulin therapy derived from the blood plasma of people who have recovered from COVID-19. It offers a standardised dose of antibodies and doesn’t need to be limited to patients with matching blood types. If the trails are successful we can expect a positive solution for the Research and Development of the medicinal field. The treatment involves infusion into patient’s blood plasma harvested from those who have recovered from COVID-19. It is hoped to contain antibodies that fight against the virus.
Also Read:
China asserts new statements regarding the emergence of the virus
Corona continues to wreak havoc in the country, 926 deaths in last 24 hours
Corona hit Bengali actor Soumitra Chatterjee in critical condition, admitted in ICU